Cuba Cyclotron Project: multidisciplinary work and the will of a government
Main Article Content
Abstract
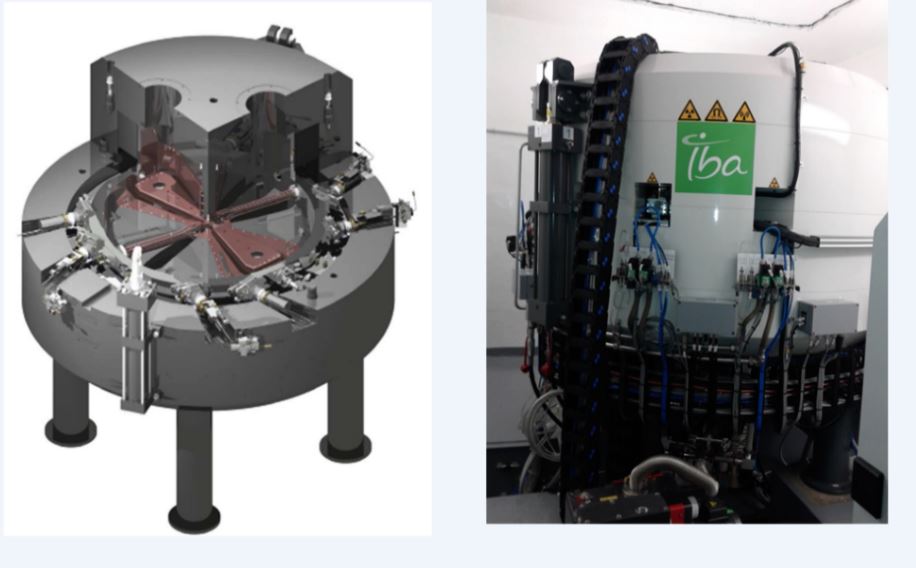
In the context of the project for the Introduction of Technologies for the Diagnosis and Treatment of cancer in our country, the Cuban Cyclotron Project was carried out, which included the structural design of the building where a medium energy particle accelerator is installed and the rest of equipment and facilities for the production of positron-emitting radiopharmaceuticals, the preparation of the production plan and the development of various stages until the start of operations. It is the first time in Cuba that a center for the production of radiopharmaceuticals of this type is set up, which is subject to compliance with specific radiological safety requirements and good practices so that the facility is safe and sustainable in the future. This paper presents the experience carried out over ten years to design and build a production center that meets the production expectations of the Ministry of Public Health to improve early diagnosis capabilities for cancer and other chronic non-communicable diseases. As a result, a facility has been built in Havana where the first cyclotron in Cuba was launched at the Specialized Center for Diagnosis and Therapy, which has a production capacity to distribute radiopharmaceuticals to the capital and other provinces of the country; as well as export to countries in the region.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
La Revista Nucleus solo aceptará contribuciones que no hayan sido previamente publicados y/o procesados, por otra publicación. Cualquier violación ese sentido será considerada una falta grave por parte del autor principal lo cual será objeto valoración por parte del Consejo Editorial, el cual dictaminará al respecto.
References
[2]. International Atomic Energy Agency (IAEA). Cyclotron produced radionuclides: principles and practice. Technical Reports Series No. 465. Vienna : IAEA, 2008.
[3]. International Atomic Energy Agency (IAEA). Alternative radionuclide production with a cyclotron. IAEA Radioisotopes and Radiopharmaceuticals Reports No. 4. Vienna : IAEA, 2021.
[4]. PÉREZ PIJUÁN S. Centro de Isótopos: continuidad, presencia y proyecciones en su Aniversario 15. Nucleus. 2012; (52): 3-8.
[5]. GUERRERO CANCIO MC y ROMERO PÉREZ TC. Introducción de tecnologías para el diagnóstico y el tratamiento del cáncer en Cuba. Nucleus. 2016; (60): 8-12.
[6]. International Atomic Energy Agency (IAEA). Cyclotron produced radionuclides:guidelines for setting up a facility. Technical Reports Series No. 471. Vienna : IAEA, 2009.
[7]. GARCÍA REYES L, MORIN ZORRILLA J, CRUZ ARENCIBIA J. Selección de un ciclotrón para la producción de radionúclidos de uso en medicina nuclear. Experiencia cubana. Nucleus. 2017; (62): 29-33.
[8]. International Atomic Energy Agency (IAEA). Cyclotron produced radionuclides: Principles and pratice.Technical Reports Series No. 465. Vienna: IAEA, 2008.
[9]. Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos (CECMED). Directrices sobre Buenas Prácticas de fabricación de productos farmacéuticos. Regulación No. 16-2012. CECMED, 2012.
[10]. Ministerio de Ciencia, Tecnología y Medio Ambiente (CITMA). Reglamento sobre Notificación y Autorización de prácticas y actividades asociadas al empleo de fuentes de radiaciones ionizantes. Resolución No. 334/2011. CITMA, 2011.
[11]. CITMA-MINSAP. Reglamento "Normas básicas de seguridad radiológica". Resolución conjunta, 2002.
[12]. Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos (CECMED). Resolución No. 1- 2018. CECMED, 2018.