Risk analysis in the production of freeze-dried kits for technetium-99m labeling of monoclonal antibodies
Main Article Content
Abstract
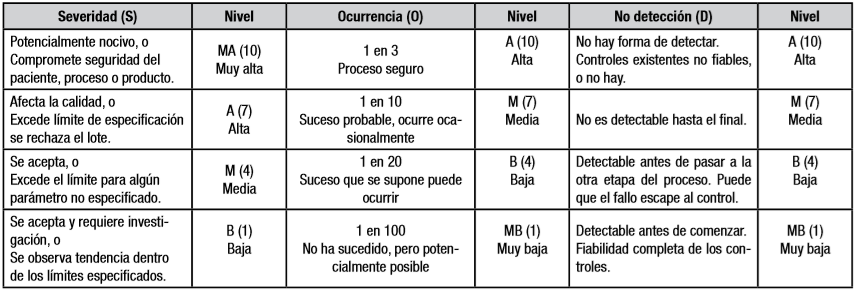
The obtainment of the Sanitary License for Pharmaceutical Operations requires the risks analysis of quality in the manufacturing process of each product. The main aim of the present work was to apply this evaluation to the production process of freeze-dried kit for labeling with Technetium-99m of monoclonal antibodies, which are developed in the Isotope Center. The failure modes and effects analysis technique and the Cuban code SECURE-MR-FMEA version 3.0 were used. The lifting of the sub-processes, stages and failure modes was carried out through brainstorming. A task group of experts reconciled their results and determined the most influential causes. The model consists of ten sub-processes, 28 stages, and 40 failure modes. Of the 98 possible combinations, 39 Sub-Process-Stage-Failure Mode-Cause ones were identified, with a risk priority number (RPN) ≥100 and a severity index (ISev) ≥7. The most contributing sub-processes were: manufacturing, conditioning and sanitizing of the clean area, preparation of glassware and materials, and the obtaining of the water for injection. The most important causes were: qualification and training of the staff; non-compliance with practices, protocols, procedures or standards; and the use of qualified equipment. The results of the sensitivity analysis showed that the solution of these causes makes possible a significant reduction of the risks of the manufacture process and, thus, contributes the compliance with good manufacturing practices, with the priority of actions and resources in these directions.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
La Revista Nucleus solo aceptará contribuciones que no hayan sido previamente publicados y/o procesados, por otra publicación. Cualquier violación ese sentido será considerada una falta grave por parte del autor principal lo cual será objeto valoración por parte del Consejo Editorial, el cual dictaminará al respecto.
References
[2]. PEÑA Y, PERERA A, BATISTA JF. Immunoscintigraphy and Radioimmunotherapy in Cuba: Experiences with Labeled Monoclonal Antibodies for Cancer Diagnosis and Treatment (1993-2013). MEDICC Review. 2014; 16 (3-4): 55-60.
[3]. Centro para el Control Estatal de Equipos y Medicamentos (CECMED). Resolución CECMED No. 155/2012. Aprueba la Guía de administración de riesgos a la calidad.
[4]. OJEDA Y, HEYNNGNEZZ L, GARCÍA J, VALDÉS Y, et. al. Aplicación del análisis de riesgo en la preparación de soluciones para producción de Quimi-Hib®. VacciMonitor. 2013; 22(2): 19-23. Disponible en: www.finlay.sld.cu/vaccimonitor.htm.
[5]. Oficina Nacional de Normalización. Sistemas de Gestión de la Calidad. Requisitos. NC ISO 9001. La Habana, 2015. [consulta: 10/10/2019]. Disponible en: http://www.nc.cubaindustria.cu.
[6]. Oficina Nacional de Normalización. Gestión del riesgo-principios y directrices. NC-ISO 31000, 2019. [consulta: 10/10/2019]. Disponible en: http://www.nc.cubaindustria.cu.
[7]. TORRES VALLE A. Programa de análisis de riesgo basado en matriz de riesgo y FMEA. Manual de Usuario SECURE-MR-FMEA 3.0. La Habana, 2017.
[8]. GARCÍA J, SANTANA Z, ZUMALACÁRREGUI L, QUINTANA M, et. al. Aplicación del análisis de riesgo a la producción de proteínas recombinantes expresadas en Escherichia coli. VacciMonitor. 2012; 21(2): 35-42. Disponible en: www.finlay.sld.cu/vaccimonitor.htm.
[9]. AMADOR BALBONA Z, TORRES VALLE A. Causas básicas de fallos aplicadas al análisis de riesgo en práctica médicas con radiaciones ionizantes [Internet]. Revista Cubana de Salud y Trabajo 20(2):11-8 2019 [consulta: 03/11/2019]. Disponible en: http://www.revsaludtrabajo.sld.cu/index.php/revsyt/article/view/99.
[10]. Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos (CECMED). Buenas prácticas farmacéuticas. Sistema regulador en Cuba. Segunda edición. La Habana: CECMED, 2017.
[11]. GUTIÉRREZ PULIDO H. Calidad total y productividad. México. 3era Edición. McGraw-Hill/Interamericana Editores, S.A. de C.V., 2010.
[12]. DA SILVA TEIXEIRA F, DE ALMEIDA C, SAIFUL HUQ M. Failure mode and effect analysis based risk profile assessment for stereotactic radiosurgery programs at three cancer centers in Brazil. Medical Physics. 2016; 43(1): 171-8.
[13]. ROY S, RUITBERG C, SETHURAMAN A. Troubleshooting During the Manufacture of Lyophilized Drug Product- Being Prepared for the Unexpected [Internet]. [consulta: 04/02/2021]. Disponible en: www.americanpharmaceuticalreview.com/Featured-Articles/126958.
[14]. Assure Batch Uniformity for Freeze-Dried Products. [Internet]. [consulta: 04/02/2021]. Disponible en: https://www.pharmamanufacturing.com/articles/2005/191.
[15]. SALAZAR MACIAN R. Problemas tecnológicos en la fabricación de medicamentos. Apuntes sobre tecnología farmacéutica. Barcelona, 2015. [consulta: 05/03/2021]. Disponible en: http://hdl.handle.net/2445/68462.
[16]. COOKE D, DUBETZ M, HESHMATI R, IFTODY S, et. al. A reference guide for learning from incidents in radiation treatment. Alberta Heritage Foundation for Medical Research, 2006.