Production of [18F]Fludeoxyglucose in Cuba
Main Article Content
Abstract
With the implementation of the technology in Cuba for manufacture of [18F]Fludeoxyglucose ([18F]FDG), it is incurred for the first time in the country in the production of positronic radiopharmaceuticals obtained from cyclotron.
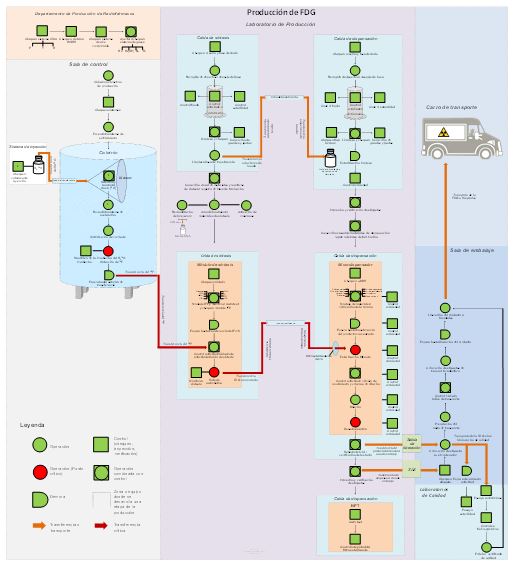
This paper presents the productive flow design of [18F]FDG manufacture at the Specialized Center of Diagnosis and Therapy based on the guidelines established by the Good Manufacturing Practices and the Basic Norms of Radiological Security. In addition, the consistency assessment in production batches is shown, information that complemented the process validation. As a results was obtained that the production process, according to the designed flow, is able to consistently provide an injectable pharmaceutical product of the required quality and that meets its specifications.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
La Revista Nucleus solo aceptará contribuciones que no hayan sido previamente publicados y/o procesados, por otra publicación. Cualquier violación ese sentido será considerada una falta grave por parte del autor principal lo cual será objeto valoración por parte del Consejo Editorial, el cual dictaminará al respecto.
References
[2] Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos (CECMED). Buenas Prácticas de Producción de Radiofármacos. Regulación No. 16-2012. Anexo no. 05. CECMED, 2014.
[3] Pharmaceutical inspection convention- Pharmaceutical inspection co-operation scheme. Guide to good manufacturing practice for medicinal products. Annexes. Geneva: PIC/S Secretariat, 2017.
[4] United States Pharmacopeia (USP). Farmacopea de los Estados Unidos de América. Cap 823. USP 40 NF35 ed. Vol. 1. 817-828.
[5] International Atomic Energy Agency (IAEA). Cyclotron produced radionuclides: guidance on facility design and production of fluorodeoxyglucose (FDG). Vienna: IAEA, 2012.
[6] Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos (CECMED). Regulación no. 16-2012 Directrices sobre Buenas Prácticas de Fabricación de productos farmacéuticos. CECMED, 2012.
[7] QUIRÓS AR. Radiofármaco [18F]FDG un aporte novedoso para el diagnóstico de cáncer en el país. Revista Médica de la Universidad de Costa Rica. 2015; 9(2). Disponible en: https://revistas.ucr.ac.cr/index.php/medica/article/view/22002/22184.
[8] YU S. Review of 18F-FDG synthesis and quality control. Biomed Imaging Interv J. 2006; 2(4): e57.
[9] SCHUBIGER PA, LEHMANN L, FRIEBE M. PET chemistry the driving force in molecular imaging. J Nucl Med. 2007; 48(10): 1750.
[10] United States Pharmacopeia (USP). Farmacopea de los Estados Unidos de América. Monografía Fludesoxiglucosa F 18, Inyección. USP 40 NF-35 ed. Vol. 2. 4673.
[11] LOVELESS VS. Quality control of compounded radiopharmaceuticals. The University of New Mexico Health Sciences Center, 2009. Disponible en: https://pharmacyce.unm.edu/nuclear_program/freelessonfiles/vol15lesson3.pdf.