Applications of amniotic membranes in Cuba: experiences and perspectives
Main Article Content
Abstract
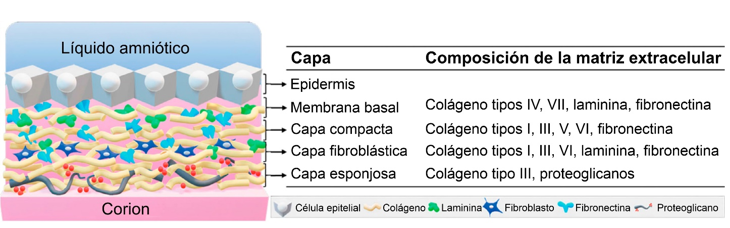
The healing, pain relieving effect and antimicrobial properties of amnion grafts have been reported in the scientific literature since the beginning of the 20thcentury. Amniotic membrane (AM) is ideal for clinical and advanced therapies. It facilitates the growth, adhesion, differentiation, and migration of epithelial cells. It is an ideal biocompatible, biological scaffold for regenerative medicine applications and advances therapies.
A validated methodology for the production of radio sterilized amniotic membrane as a medical device has been implemented at the Centre for Applied Technologies and Nuclear Development (CEADEN) for more than 20 years. According to actual Cuban standards, this product is a class III medical devise, because of its origin. It has-been employed in several ophthalmological and dermatological pathologies, with promising results. Due to its outstanding properties, AM is highly in demand, allowing a faster recovery of patients. In addition, it is relatively easy to obtain with low-cost production, transforming it into a valuable alternative to other products. Recently, CEADEN participated in project supported by the International Atomic Energy Agency (IAEA) focused on scaffolds and tissue engineering. In addition, it is developing a research project financed by the Agency for Nuclear Energy and Advanced Technologies (AENTA) with the aim of obtain and apply AM as a biocompatible scaffold.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista. Bajo esta licencia el autor será libre de:
- Compartir — copiar y redistribuir el material en cualquier medio o formato
- Adaptar — remezclar, transformar y crear a partir del material
- El licenciador no puede revocar estas libertades mientras cumpla con los términos de la licencia
Bajo las siguientes condiciones:
- Reconocimiento — Debe reconocer adecuadamente la autoría, proporcionar un enlace a la licencia e indicar si se han realizado cambios. Puede hacerlo de cualquier manera razonable, pero no de una manera que sugiera que tiene el apoyo del licenciador o lo recibe por el uso que hace.
- NoComercial — No puede utilizar el material para una finalidad comercial.
- No hay restricciones adicionales — No puede aplicar términos legales o medidas tecnológicas que legalmente restrinjan realizar aquello que la licencia permite.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).
La Revista Nucleus solo aceptará contribuciones que no hayan sido previamente publicados y/o procesados, por otra publicación. Cualquier violación ese sentido será considerada una falta grave por parte del autor principal lo cual será objeto valoración por parte del Consejo Editorial, el cual dictaminará al respecto.
References
[2]. LEAL MARTÍN S, THOMAS K, NICOLA H, et. al. Human Amniotic Membrane: a review on tissue engineering, application, and storage. J Biomed Mater Res. 2021; 109(18):1198-1215.
[3]. DAVIS JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910; 15:307.
[4]. GUTIERREZ S, ALSINA M, SAMPIETRO L, PEDREGOSA S, AYALA P. Estudio coste-beneficio del trasplante de membrana amniótica para úlceras venosas de extremidades inferiores refractarias a tratamiento convencional. Janssen Inmunology. 2011; 102(4): 284-288.https://doi.org/10.1016/j.ad.2011.01.003.
[5]. KAIRIYAMA E, MARTINEZ ME, SÁNCHEZ E, OTERO IM. Overview of radiation and tissue banking in Latin America. Cell and Tissue Bank. 2018; 19(2): 249-257.
[6]. OTERO IM, RODRÍGUEZ D, YI D, SÁNCHEZ E. Apósitos de membrana amniótica radioesterilizados: una alternativa factible para el sistema de salud cubano. Convención Internacional de salud: Cuba-Salud 2015.ID: 1534.
[7]. Organización Panamericana de Salud (OPS). Causas principales de mortalidad y pérdidas en salud de nivel regional, subregional y nacional en la Región de las Américas, 2000-2019. Organización Panamericana de Salud (OPS), 2021.
[8]. MIQUET LM, VÁZQUEZ CL, RODRÍGUEZ RG, TAMARGO TO. Comportamiento del peso corporal durante la atención del paciente en una unidad de quemados. Revista Cubana de Alimentación y Nutrición. 2013; 23(1): 1561-2929.
[9]. ORTEGA JM, CHAVES MJ, SALGADO A, PÉRES S. La membrana amniótica en oftalmología: del recubrimiento-injerto a la ingeniería tisular. Rev Esp Inv Oftalmol 2014; 4(2): 117-122.
[10]. HASMAD H, YUSOF MR, MOHD RAZI ZR, et. al. Human amniotic membrane with aligned electrospun fiber as scaffold for aligned tissue regeneration. Tissue Eng Part C Methods. 2018; 24(6): 368-378. https://doi.org/10.1089/ten.tec.2017.0447.
[11]. SABOURI L, FARZIN A, KABIRI A, et. al. Mineralized human amniotic membrane as a biomimetic scaffold for hard tissue engineering applications. ACS Biomater. Sci. Eng. 2020; 6(11): 6285-6298. https://doi.org/10.1021/acsbiomaterials.0c00881.
[12]. NEJAD RA, HAMIDIEH AA, AMIRKHANI MA, SISAKHT MM. Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta, 2021; 103: 104-119. https://doi.org/10.1016/j.placenta.2020.10.026
[13]. FÉNELON M, CATROS S, MEYER C, et. al. Applications of human amniotic membrane for tissue engineering. Membranes. 2021; 11(6): 387. https://doi.org/10.3390/membranes11060387.
[14]. HASHEMI SS, MOHAMMADI AA, MOSHIRABADI K, ZARDOSHT M. Effect of dermal fibroblasts and mesenchymal stem cells seeded on an amniotic membrane scaffold in skin regeneration: a case series. J Cosmet Dermatol. 2021; 20(1)): 1-8. https://doi.org/10.1111/jocd.14043.
[15]. NOURI M, EBRAHIMI M, BAGHERI T, et. al. Healing effects of dried and acellular human amniotic membrane and Mepitelas for coverage of skin graft donor areas: a randomized clinical trial. Bull Emerg Trauma. 2018; 6(3): 1955-200. https://doi.org/10.29252/beat-060302.
[16]. FITRIANI N, WILAR G, NARSA AC, et. al. Application of amniotic membrane in skin regeneration. Pharmaceutics 2023; 15(3): 748. https://doi.org/10.3390/pharmaceutics15030748.
[17]. Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos. (CECMED). Evaluación estatal de equipos y dispositivos médicos. Regulación E 86-16. La Habana: CECMED, 2016.
[18]. SANTANA MC, ESQUIVEL M, HERRERA VR, et. al. Atención a la salud materno-infantil en Cuba: logros y desafíos. Rev Panam Salud Publica. 2018; 42: e27. https://doi.org/10.26633/RPSP.2018.27.
[19]. NC-ISO 13485:2018. Equipos Médicos –Sistemas de Gestión de la Calidad-Requisitos para propósitos reguladores. 2018
[20]. ÁLVAREZ SALDÍAS I. Guía para la operación de bancos de tejidos. Cartago, Costa Rica: Editorial Tecnológica de Costa Rica, 2013.
[21]. KAIRIYAMA E. Código de prácticas para la esterilización por irradiación de tejidos humanos para uso clínico: requisitos para la validación y control de rutina. Cartago, Costa Rica: Editorial Tecnológica de Costa Rica, 2013.
[22]. OTERO IM, BARRERA, L, RODRÍGUEZ D. Sterilization of amnion grafts under code of practice of radio-sterilization application. Nucleus. 2004; (35): 48-51.
[23]. Oficina Nacional de Estadística e Información de la República de Cuba (ONEI). Anuario Demográfico de Cuba 2022. La Habana: ONEI, Julio 2023.
[24]. ORELLANA A, OTERO IM, RAPADO M, et. al. Evaluación clínica de las tecnologías combinadas de láser-amnio y láser-hidrogel para el tratamiento de úlceras venosas de miembros inferiores. Actas de Congreso Cuba-Salud. 2015. ID:1476.
[25]. ARAGONÉS B. Utilización de la membrana amniótica radioesterilizada en cirugía de pterigium. Rev Cubana Oftalmol. 2006; 19(2).
[26]. FERNÁNDEZ K, GÓMEZ Z, CASTILLO A, et. al. Autoinjerto conjuntival y membrana amniótica en la cirugía del pterigión primario. Rev Cubana Oftalmol. 2012; 25(2).
[27]. HERNÁNDEZ Y, PÉREZ Z, LEÓN Y, et. al. Recubrimiento conjuntival en afecciones corneales. Rev Cubana Oftalmol. 2018; 31(4).
[28]. RODRÍGUEZ DE Paz U. Autoinjerto limboconjuntival con membrana amniótica en la insuficiencia límbica total unilateral. Rev Cubana Oftalmol. 2014; 27(4): 640-646.
[29]. FERDOUS K, MASARU T, SHEIKH A. Fabrication of polymeric biomaterials: a strategy for tissue engineering and medical devices. Journal of Materials Chemistry B. 2015; 3: 8224- 8249.
[30]. TAGHIABADI E, NASRI S, SHAFIEYAN S, et. al. Fabrication and characteriza¬tion of spongy denuded amniotic membrane based scaffold for tissue engineering. Cell J. 2015; 16(4): 476-487.
[31]. SRIPRIYA R, KUMAR R. Denudation of human amniotic membrane by a novel processand its characterizations for biomedical applications. Prog Biomater. 2016; 5:161-172. https://doi.org/10.1007/s40204-016-0053-7.
[32]. ELKHENANY H, EL DERBY A, ELKODOUS MA, et. al. Applications of the amniotic membrane in tissue engineering and regeneration: the hundred year challenge. Stem Cell Research & Therapy. 2022; 13:8.https://doi.org/10.1186/s13287-021-02684-0.
[33]. CHEN YJ, CHUNG MC, JANE YAO CC, et. al. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials, 2012; 33: 455-463.
[34]. JERMAN UD, VERANI P, KREFT ME. Amniotic membrane scaffolds enable the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of native urothelium. Tissue Engineering: Part C. 2014; 20(4): 317-327. https://doi.org/10.1089/ten.tec.2013.0298.
[35]. AMENSAG S, MCFETRIDGE PS. Rolling the human amnion to engineer laminated vascular tissues. Tissue Engineering: Part C. 2012; 18(11): 903-912. https://doi.org/10.1089/ten.tec.2012.0119