CIENCIAS
NUCLEARES
Radiation induced graft copolymerization of acrylamide onto poly (3-hydroxybutyrate)
Copolimerización por injerto radioinducido de acrilamida en el poli (3-hidroxibutirato)
Maykel González
Torres1, Manuel Rapado Paneque2, Mayte Paredes Zaldívar2, Sonia Altanés
Valentín2, Gisela Barrera González2
1Instituto de Ciencia y Tecnología de Materiales (IMRE), Universidad de La Habana, Cuba
2Centro de Aplicaciones Tecnológicas y Desarrollo Nuclear (CEADEN)
Calle 30 No 502 e/ 5ta Ave. y 7ma, Playa, Ciudad de La Habana, Cuba
ABSTRACT
The graft copolymer poly (3-hydroxybutyrate)-g- polyacrylamide [P (HB-g-AAm)] was synthesized by radiation induced graft copolymerization of acrylamide onto poly (3-hydroxybutyrate). The study was conducted by the simultaneous irradiation method. The structure of [P (HB-g-AAm)] was identified by Fourier Transform Infrared (FTIR) spectroscopy. Thermal behavior of the graft copolymer was also studied by Thermal Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC). According to the results, FTIR studies showed new signals (stretching-N-H) as an important evidence of grafting. The grafting degree, determined by ATG, was 10% and changes in thermodynamic parameter obtained from the DSC were detected. Such changes show a decrease in crystallinity and an increase in the glass transition temperature. These results demonstrate that the gamma radiation-induced graft copolymerization reaction of acrylamide onto PHB was successively achieved.
RESUMEN
En el trabajo se realizó la síntesis del copolímero por injerto poli (3-hidroxibutirato)-g- poliacrilamida [P (HB-g-AAm)] por copolimerización de injerto radioinducido del monómero acrilamida en el PHB. El estudio se llevó a cabo por el método de la irradiación simultánea. La estructura del copolímero se identificó por espectroscopía Infrarrojo por Transformada de Fourier (FTIR). Además, se realizó el estudio térmico del compuesto obtenido por análisis termogravimétrico (TGA) y calorimetría diferencial de barrido (DSC). De los resultados se encontraron señales (vibraciones de valencia -N-H) por FTIR que constituyen una importante evidencia de la reacción de injerto. El grado de injerto determinado por ATG fue de 10% y se detectaron cambios en los parámetros termodinámicos estudiados por DSC, que muestran una disminución de la cristalinidad y un aumento de la temperatura de transición vítrea. Los resultados demostraron la formación del copolímero por injerto inducido por radiaciones gamma.
Key words: acrylamide, graft polymers, irradiation procedures, fourier transform spectrometers, thermal gravimetric analysis, copolymers, radiation doses, gamma radiation
INTRODUCCIÓN
Poly (3-hydroxybutyrate)
(PHB) has received considerable attention in the last decade as biodegradable
and biocompatible biomaterial. PHB is a thermoplastic material with physical
properties similar to polypropylene (PP) [1]. Its various properties make it
suitable for a variety of applications in the field of medicine [2]. However,
the PHB homopolymer main drawbacks are its relatively hydrophobicity, brittleness
and thermal instability. It is known that these problems can be solved by the
incorporation of new molecules to the PHB backbone by graft copolymerization.
On the other hand, polyacrylamide has shown to be «biocompatible»,
although some controversy exists with respect to its blood biocompatibility
[3, 4]. The covalent grafting of hydrophilic polymers onto hydrophobic substrates
is widespread. Graft polymerization is a well-known method to modify the chemical
properties of polymers for specific application [5-9].
Lee et al studied graft copolymerization of acrylamide onto poly (hydroxybutyrate-co-hydroxyvalerate)
film to test the application of the grafted film on permselectivity [10].
Hydrophilic monomers, including methyl methacrylate (MMA), 2-hydroxyethyl methacrylate
(HEMA) and acrylic acid (AAc) have been grafted onto PHB and its copolymers
P (HB-co-HV) through radiation induced graft copolymerization [11-13]. Other
monomer such as styrene (St) was grafted by using the simultaneous radiation
and pre-irradiation techniques from a 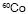 g-ray source [14]. In addition Hu et al reported the graft copolymerization
of isoprene (Ip) obtained by directly irradiating the polymer immersed in isoprene
solution [15].
g-ray source [14]. In addition Hu et al reported the graft copolymerization
of isoprene (Ip) obtained by directly irradiating the polymer immersed in isoprene
solution [15].
In previous paper the radiation induced graft copolymerization of vinyl acetate onto PHB was performed [16, 17]. The study reported here is concerned with the radiation induced graft copolymerization of typical acrylamide onto PHB by the simultaneous irradiation method. Simultaneous irradiation is the simplest irradiation technique for preparation of graft copolymers. In this method a polymer backbone is irradiated in the presence of a monomer available in different forms: vapor, liquid or in bulk solution. The purpose of grafting AAm onto PHB is to improve its hydrophilic characteristics. The product obtained in this work has not been reported before by any authors. It was characterized by means of several techniques to verify the graft copolymer synthesis.
MATERIALS AND METHODS
Materials
Poly (3-hydroxybutyrate)
(PHB) was available from the Institute of Research and Technology, Biotechnology
Section (Brazil). It was purified by precipitation in ethanol from chloroform
solutions. The molecular weights were determined by GPC at room temperature
with a PUMP 64 HPLC (KNAUER), equipped with a series of five columns PL-Gel
and KNAUER differential refractometer. Tetrahydrofuran (THF) solvent was used
at 1 mL/min flow rate, and it was also used injection volumes of 20 µL.
Polystyrene standards with low polydispersity were used to generate a calibration
curve. The results were: 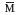 n
= 27000,
n
= 27000, 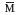 w
= 60000;
w
= 60000; 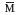 z
= 98000; Polydispersity = 2.22.
z
= 98000; Polydispersity = 2.22.
Acrylamide was available from Merck (Ge) and was previously purified by recrystallization
method.
Graft copolymer synthesis (P (HB-g-AAm))
The irradiation
experiment was carried out in glass sealed vacuum ampoules at room temperature.
PHB (200 mg) was immersed in AAm (1g) and 1 mL of the solvent (acetone). The
mixture was subjected to 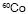
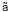 -ray
at a dose rate of 1. 62 kGy/h and a dose of 10 kGy. The grafted PHB so obtained
was extracted in a Soxhlet apparatus with ethanol and acetone for 72 h, to remove
any acrylamide monomer and PAAm homopolymer obtained as collateral products.
The copolymer P (HB-g-AAm) was dried under vacuum, at 40 C, to constant weight.
-ray
at a dose rate of 1. 62 kGy/h and a dose of 10 kGy. The grafted PHB so obtained
was extracted in a Soxhlet apparatus with ethanol and acetone for 72 h, to remove
any acrylamide monomer and PAAm homopolymer obtained as collateral products.
The copolymer P (HB-g-AAm) was dried under vacuum, at 40 C, to constant weight.
The following equation to determine the degree of grafting (W (%)) in the graft copolymerization reactions is often used:
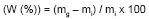
Where 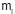 (g) is the initial weight of the original base backbone polymer and mg (g) is
the graft copolymer weight after grafting. This variable is an alternative to
elucidate the graft % by means of the mass increase.
(g) is the initial weight of the original base backbone polymer and mg (g) is
the graft copolymer weight after grafting. This variable is an alternative to
elucidate the graft % by means of the mass increase.
Characterization
Thermal analysis
The study of the
graft copolymer (P (HB-g-AAm)) obtained by radiation induced graft copolymerization
was conducted by Thermal Gravimetric Analyses (TGA) in a Q500 TA Instruments,
at a heating rate of 10°C/min at nitrogen atmosphere. The samples weight
was of 5-15 mg and the temperature range of 50- 600°C at atmosphere.
Thermal properties
were also investigated using Differential Scanning Calorimetry (DSC), on a TA
Instrument 2920 under nitrogen atmosphere. In the DSC experiments, at first,
at the heating rate of 10°C/min, the sample was heated from -30 to 180°C
(first heating scan), and keep for few minutes at 180o C. After eliminating
the thermal history, the sample was cooled to -30°C (-10°C/min) and
finally the sample was heated from -30 to 180°C at the heating rate of 10°C/min
(second heating scan). The enthalpy and the transition temperatures were determined
from exothermic and endothermic peaks in the second run.
FTIR studies
Fourier Transform Infrared (FTIR) transmittance spectra (520 scans, 4 cm- resolution, wave number range 400-4000 cm-), were recorded using a Bruker Vertex 70 FTIR Spectrometer. All spectra were baseline corrected. The sample was prepared in KBr pellets. The study was performed with the diffuse reflectance accessory (EasiDiff).
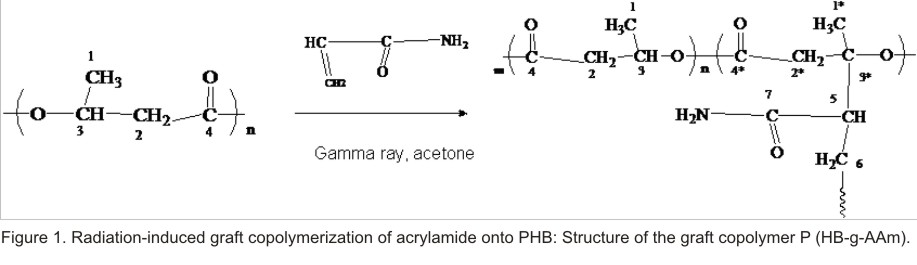
RESULTS AND DISCUSSION
Figure 1 show the
radiation induced graft copolymerization reaction of acrylamide onto PHB. The
simultaneous irradiation technique with  gamma-ray was used. The dose rate was 1. 62 kGy/h and the dose was 10 kGy at
room temperature. The selection of the source, dose rate and dose used are based
on the results obtained in the radiation induced graft copolymerization of other
monomers onto PHB synthesized by the authors. For instance the P (HB-g-VAc).
These parameters have also been used in the syntheses of the graft copolymers
found in the literature [11-15]. It is important to mention here that generally,
higher irradiation doses lead to higher degrees of grafting. This is due to
the enhancement of free radical formation. But the graft copolymer is always
obtained at doses over 5 KGy. We have chosen 10 KGy because it was verified
experimentally that this value is appropriate to be used.
gamma-ray was used. The dose rate was 1. 62 kGy/h and the dose was 10 kGy at
room temperature. The selection of the source, dose rate and dose used are based
on the results obtained in the radiation induced graft copolymerization of other
monomers onto PHB synthesized by the authors. For instance the P (HB-g-VAc).
These parameters have also been used in the syntheses of the graft copolymers
found in the literature [11-15]. It is important to mention here that generally,
higher irradiation doses lead to higher degrees of grafting. This is due to
the enhancement of free radical formation. But the graft copolymer is always
obtained at doses over 5 KGy. We have chosen 10 KGy because it was verified
experimentally that this value is appropriate to be used.
The structure shown in the figure suggested that the graft occurs through the tertiary PHB carbon. This can be considered because of previous studies [18]. Sevilla et al reported that the tertiary radical formed by the exposition of PHB to gamma-radiation would be favored with respect to the rest possible radicals formed. In our previous studies, the P (HB-g-VAc) was reported [19]. Spectroscopic studies showed that quaternary substituted carbons were obtained. This result is in agreement with the propose structure given for P (HB-g-AAm). On the other hand, Grushevskaya et al reported that acetone is the more convenient solvent to be used in the radiation induced graft copolymerization of acrylamide onto PE. From this finding acetone was used as diluent in this work [20].
FTIR
FTIR studies were
performed in order to confirm the syntheses of a graft copolymer. Evidence of
grafting was obtained by using this technique. Figure 2 shows the transmittance
mode FTIR spectra of pure PHB and P (HB-g-AAm). From the IR spectra, it was
obvious that PHB showed the bands at 1185, 1228 and 1279  characteristic of
characteristic of  (C-O-C) and the band at 1382
(C-O-C) and the band at 1382 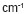 characteristic of
characteristic of 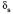 (
(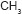 ).
In the ester carbonyl region, it can be considered the band of the C=O stretching
(1729
).
In the ester carbonyl region, it can be considered the band of the C=O stretching
(1729 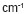 ). All this
mentioned signals are common for both materials. However in the graft copolymer,
apart of the previous peaks, four additional peaks were present. Two peaks at
3300 cm- (broad peak) and 3182 cm- were for the
). All this
mentioned signals are common for both materials. However in the graft copolymer,
apart of the previous peaks, four additional peaks were present. Two peaks at
3300 cm- (broad peak) and 3182 cm- were for the 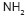 groups, corresponding to symmetrical and antisymmetrical stretching -N-H bonds.
The peak position at 1676 cm- is probably caused by the amide carbonyl group
and the 1608 cm- peak represents the adsorption of
groups, corresponding to symmetrical and antisymmetrical stretching -N-H bonds.
The peak position at 1676 cm- is probably caused by the amide carbonyl group
and the 1608 cm- peak represents the adsorption of 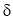 (NH). The absorption frequency of carbonyl stretching vibrating of the amide
group is well separated from that of the ester group. The new peaks observed
are a strong evidence of grafting and clearly indicate the formation of a graft
copolymer.
(NH). The absorption frequency of carbonyl stretching vibrating of the amide
group is well separated from that of the ester group. The new peaks observed
are a strong evidence of grafting and clearly indicate the formation of a graft
copolymer.
Differential Scanning Calorimetry (DSC) investigation
Thermal properties
of the grafted PHB (figure 3) and the P (HB-g-AAm) were investigated by DSC
with the purpose to demonstrate the graft copolymer formation by means of the
differences observed among both materials. Figure 4 is the thermogram of graft
copolymer. In the DSC thermogram of the first scan was observed the fist anomaly
among both materials. The graft copolymer showed bimodal peaks at 175.11°C
and 174.46°C with respect to PHB endothermic melting (175.23°C). In
addition in the DSC of the second scan was observed that the crystallization
temperature changed its value from 97.4°C (PHB) to 95.4°C (P (HB-g-AAm))
and the enthalpies of crystallization ( 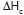 )
changed its value from 94.28 J/g (PHB) to 82.42 J/g (P (HB-g-AAm)). What is
more, in the DSC thermogram of the third scan was also observed anomalies. The
thermogram of the product showed bimodal peaks in the melting region. Surprisingly
the melting point (
)
changed its value from 94.28 J/g (PHB) to 82.42 J/g (P (HB-g-AAm)). What is
more, in the DSC thermogram of the third scan was also observed anomalies. The
thermogram of the product showed bimodal peaks in the melting region. Surprisingly
the melting point (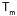 )
of the graft copolymer was three degrees up and down the value of the
)
of the graft copolymer was three degrees up and down the value of the 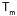 given for PHB. Consequently, the enthalpies of melting (
given for PHB. Consequently, the enthalpies of melting (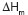 )
of the plain PHB and the graft copolymer differed in about 15 units (J/g). The
decrease of the
)
of the plain PHB and the graft copolymer differed in about 15 units (J/g). The
decrease of the 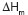 for the graft copolymer indicated that the cristallinity decreased after the
introduction of AAm chains onto the PHB backbone by radiation induced graft
copolymerization. It is of interest to mention here that the
for the graft copolymer indicated that the cristallinity decreased after the
introduction of AAm chains onto the PHB backbone by radiation induced graft
copolymerization. It is of interest to mention here that the 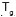 of plain PHB changed from -2°C to 9.61°C in the graft copolymer, laying
between those of the two homopolymers. This result is consistent with the fact
that is natural that the introduction of AAm chains onto PHB increases the glass
transition temperature.
of plain PHB changed from -2°C to 9.61°C in the graft copolymer, laying
between those of the two homopolymers. This result is consistent with the fact
that is natural that the introduction of AAm chains onto PHB increases the glass
transition temperature.
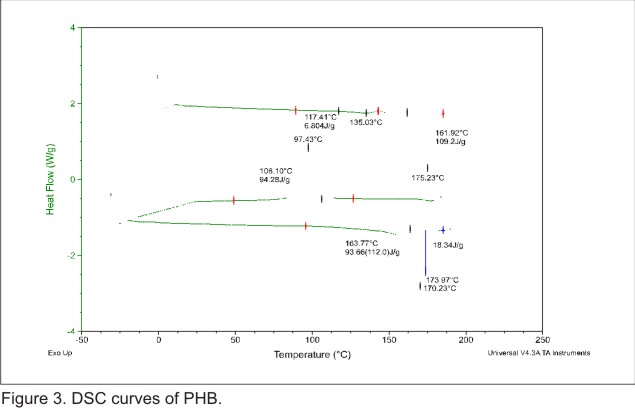
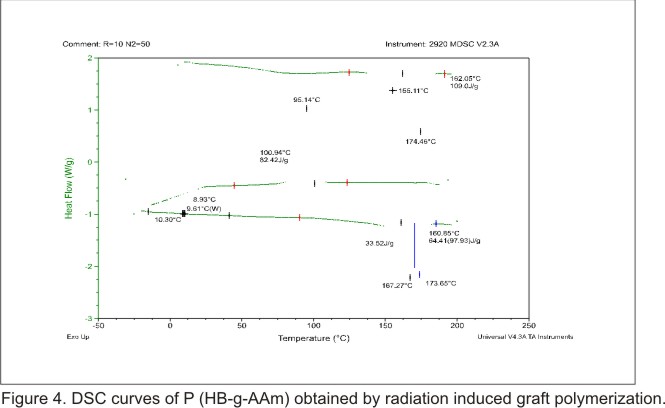
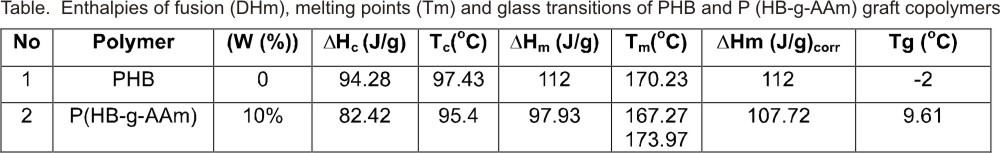
Table lists the
thermodynamic parameters obtained from the DSC thermogram of the experiment
sample. It was summarized the melting point (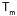 ),
the enthalpies of melting (
),
the enthalpies of melting (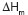 ),
the enthalpies of melting
),
the enthalpies of melting 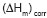 corrected by the weigh fraction of the polymer that conform the graft copolymer,
calculated according to the following equation:
corrected by the weigh fraction of the polymer that conform the graft copolymer,
calculated according to the following equation:
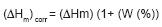
the crystallization
temperature (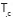 )
and the enthalpies of crystallization (
)
and the enthalpies of crystallization (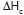 ).
From the results obtained by the DSC experiments a strong evidence of grafting
is given.
).
From the results obtained by the DSC experiments a strong evidence of grafting
is given.
Thermogravimetric analysis (TGA)
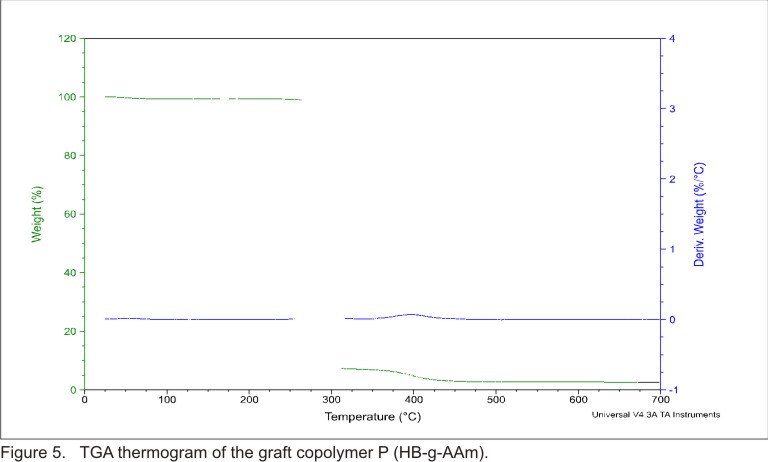
Figure 5 illustrates
TGA thermogram of the graft copolymer P (HB-g-AAm). The TGA was conducted to
show the difference in the thermal behavior of the obtained product with respect
to the base polymer (PHB) and the grafting degree calculus. The TG curve of
PHB grafted with AAm shows two steps in its smooth weight loss curve because
the decomposition temperature of PHB and AAm are not the same. The first step
represents the plain PHB material and the second step represents the grafted
PAAm. In addition, the DTGA shows two rates of weight loss (dW/dt) peaks defining
the temperature of each step. The first step at 395°C belongs to PHB characteristic
temperature. The others step at 395°C correspond to PAAm decomposition.
From figure 5 can be seen that the PHB composition in the graft copolymer can
be calculated by dividing the weight loss of the first step to the total weight
loss. This result indicates that the copolymer grafting degree (W (%)) is 10%
(This percentage represent the quantities in mass of PAAm grafted onto PHB with
respect to the total mass of the sample) (see table).
CONCLUSIONS
The radiation induced graft copolymerization reaction of AAm onto PHB has been studied by the simultaneous irradiation method. The results obtained can be summarized as follows:
1- In spite of
the PHB inactive structure, AAm can be grafted onto PHB by directly irradiating
PHB and AAm immersed in acetone solution, using a 
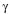 -ray
source.
-ray
source.
2- The synthesized material was characterized by means of several techniques
such as FTIR, ATG and DSC
3- FTIR studies showed new signals that strongly confirm the synthesis of P(HB-g-AAm),
such as the peaks at 3300 cm- and 3182 cm- assigned for the 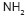 groups, corresponding to symmetrical and antisymmetrical stretching -N-H bonds.
In addition, the absorption frequency of carbonyl stretching vibrating of the
amide group is well separated from that of the ester group.
groups, corresponding to symmetrical and antisymmetrical stretching -N-H bonds.
In addition, the absorption frequency of carbonyl stretching vibrating of the
amide group is well separated from that of the ester group.
4- TGA results showed that graft degree (W (%)) in the graft copolymer is about
10% calculated by dividing the weight loss of the second step to the total weight
loss of the sample.
5- From DSC results, the melting enthalpy (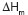 )
slightly decreased. The decrease of the
)
slightly decreased. The decrease of the 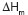 for the graft copolymer indicated that the cristallinity decreased after the
introduction of AAm chains onto the PHB backbone by radiation induced graft
copolymerization. Some experiments displayed bimodal peaks during the DSC in
the third scan, which could be caused by recrystallization processes. Consequently,
the glass transition temperature increased its value as a result of the grafting.
for the graft copolymer indicated that the cristallinity decreased after the
introduction of AAm chains onto the PHB backbone by radiation induced graft
copolymerization. Some experiments displayed bimodal peaks during the DSC in
the third scan, which could be caused by recrystallization processes. Consequently,
the glass transition temperature increased its value as a result of the grafting.
6- These results demonstrate that the radiation induced graft copolymerization
reaction of acrylamide onto PHB was successively achieved.
Acknowledgment
The authors are grateful to: Dr Ramiro Guerrero, Dr Enrique Saldívar, Silvia Solís, Blanca Huerta Guadalupe Mendez and Judith Cabello from CIQA (Centro de Investigación en Química Aplicada, México) for their assistance with TGA and DSC data collection. We would also thank to Dr Judith Percino and Dr V.M Chapela from the Benemérita Universidad Autónoma de Puebla (BUAP), Joaquín Iglesias (Glass technician specialist) from Biomat (UH-Cuba), and Dr Ricardo Aroca from the Windsor University of Canada.
REFERENCES
[1] SUDESH K, ABE H, DOI Y. Syntheses, structure and properties of polyhydroxyalkanoates: biological polyester. Prog. Polym. Sci. 2000; 5: 1503-1555.
[2] ZINN M, WITHOLT B, EGLI T. Occurrence, syntheses and bacterial application of bacterial polyhydroxyalkanoate. Advanced Drug Delivery Reviews 2001; 53: 5–21.
[3] HOFFMAN AS, BUDDY RD. The radiation grafting of acrylamide to polymer substrates in the precence of cupric ion-I. A preliminary study. Radiat. Phys. Chem. 1979; 14: 831-840.
[4] DESSOUKI AM, HEGAZY EA, EL-ASSY NB, EL-BOOHY HA. Radiation-induced graft polymerization of acrylamide: reverse osmosis properties of polyethylene-g-polyacrylamide membrane. Radiat. Phys. Chem. Int. Jnl. Radiat. Applic. Instrum, Part C. 27(6), 1986; 431-436.
[5] GRUSHEVSKAYA LN, ALIYEV RE, KABANOV VY. Radiation induced graft polymerization of acrylamide and acrylic acid onto polyethylene. Int. Jnl. Radiat. Applic. Instrum Part C. 1990; 36 (3): 475-479.
[6] GUPTA B, ANJUM N. Surface Structure of Radiation-Grafted Polyethylene-g-Polyacrylamide Films. J Appl Polym Sci. 2002; 86: 1118–1122.
[7] FAYEK SA, EL SAYED SM, EL-ARNAOUTY MB. Study the effect of gamma irradiation on optical and morphological properties of grafted low density polyethylene. Polymer Testing. 2000; (19): 435–443.
[8] DESSOUKI AM, TALLER NH, EL-BOOHY HA. Radiation-induced graft polymermization of acrylamide onto
poly (tetrafluoroethylene/hexafluoropropylene/vinylidene fluoride) (tfb) films.
Radiat. Phys. Chem. Int. Jnl. Radiat. Applic. Instrum, Part C. 1990; 36 (3): 371-375.
[9] KE Y, WANG Y, REN L, et al. Polymerization of Polyacrylamide on PHBV Films (I). Appl Polym Sci 2007; 104: 4088–4095.
[10] SUP LEE H, YOUN LEE T. Graft polymerization of acrylamide onto poly( hyd ro xyb utyrate- co- hyd ro xyvalerate) films. Polymer. 1997; 38 (17): 4505-4511.
[11] MITOMO H, ENJÔJI T, WATANABE Y, et al. Radiation induced graft polymerization of poly (3-hydroxybutyrate) and its copolymer. Pure Appl. Chem. 1995 ; A32(3): 429-442.
[12] GRØNDAHL L, CHANDLER-TEMPLE A, TRAU M. Polymeric Grafting of Acrylic Acid ontoPoly (3-hydroxybutyrate-co-3-hydroxyvalerate): SurfaceFunctionalization for Tissue Engineering Applications. Biomacromolecules. 2005: 2197-2203.
[13] WADA Y, MITOMO H, et al. Control of biodegradability of poly(3-hydroxybutyric acid) film with grafting acrylic acid and thermal remolding. J Appl Polym Sci. 2006 ; 101: 3856–3861.
[14] BAHARI, K, MITOMO, H, ENJOJI, T, et al. Radiation induced graft polymerization od styrene onto poly (3-hydroxybutyrate) and its copolymer with 3-hydroxyvalerate. Die Angewandte Makromolekulare Chemie. 1997; 250(4352): 31-44.
[15] JIANG T, HU P. Radiation-Induced Graft Polymerization of Isoprene onto Polyhydroxybutyrate. Polymer Journal, 2001; 33(9): 647-653.
[16] GONZÁLEZ M, GALEGO N, ORTIZ DEL TORO P, RAPADO M. Espectroscopia Raman del poli(3-hidroxibutirato), modificado con poliacetato de vinilo por copolimerización radioinducida. Nucleus, 2007 ; (42): 40-44.
[17] WADA Y, SEKO N, NAGASAWA N, TOMADA M, KASUYA K, MITOMO H. Biodegradability of poly (3-hydroxybutyrate) film grafted with vinyl acetate: effect of grafting and saponification Radiation Physics and Chemistry. 2007; (76): 1075-1083.
[18] CARSWELL T, HILL DJT. An electron spin resonance study of the radiation chemistry of poly (3-hydroxybutyrate). Radiat. Phys. Chem. 1995; 45(5): 737-744.
[19] GONZÁLEZ M, GALEGO N, ORTIZ DEL TORO P, RAPADO M. Radiation induced graft copolymerization of vinyl acetate onto poly (3-hydroxybutyrate). Paper summitted to JPSCH. 2008.
[20] GRUSHEVSKAYA LN, ALIYEV RE, KABANOV VY. Radiation induced graft copolymerization of acrylamide onto polyethylene. Polymer Science U.S.S.R. 1989; 31(7): 1530-1534.
Recibido: 25 de febrero de 2008.
Aceptado: 26 de marzo de 2008.
mikegcu@gmail.com, rapado@ceaden.edu.cu